In the same section
- Médecine
- Human Reproduction
- EN
- Activities
- Male fertility projects
- Ion channels and spermatogenesis
Ion channels and Spermatogenesis
- Ion channels and Spermatogenesis
Spermatogenesis is a complex developmental process involving differentiation of spermatogonial stem cells into mature spermatozoa throughout the adult life. The molecular mechanisms that control germ cell differentiation
and maturation in mammals are notoriously difficult to unravel, given the intricate anatomy and complex endo- and paracrinology of the testis. Ion channels and transporters are key elements in sperm–egg signaling, environmental sensing and are essential for fertilization, but little is known about their role in spermatogenesis. We have developed new procedure to obtain acute testis slices that will allow to study the evolution of the electrophysiological profile of germ cells during their maturation. Investigating these biological properties may provide critical insights for understanding stem cell biology and tissue development.
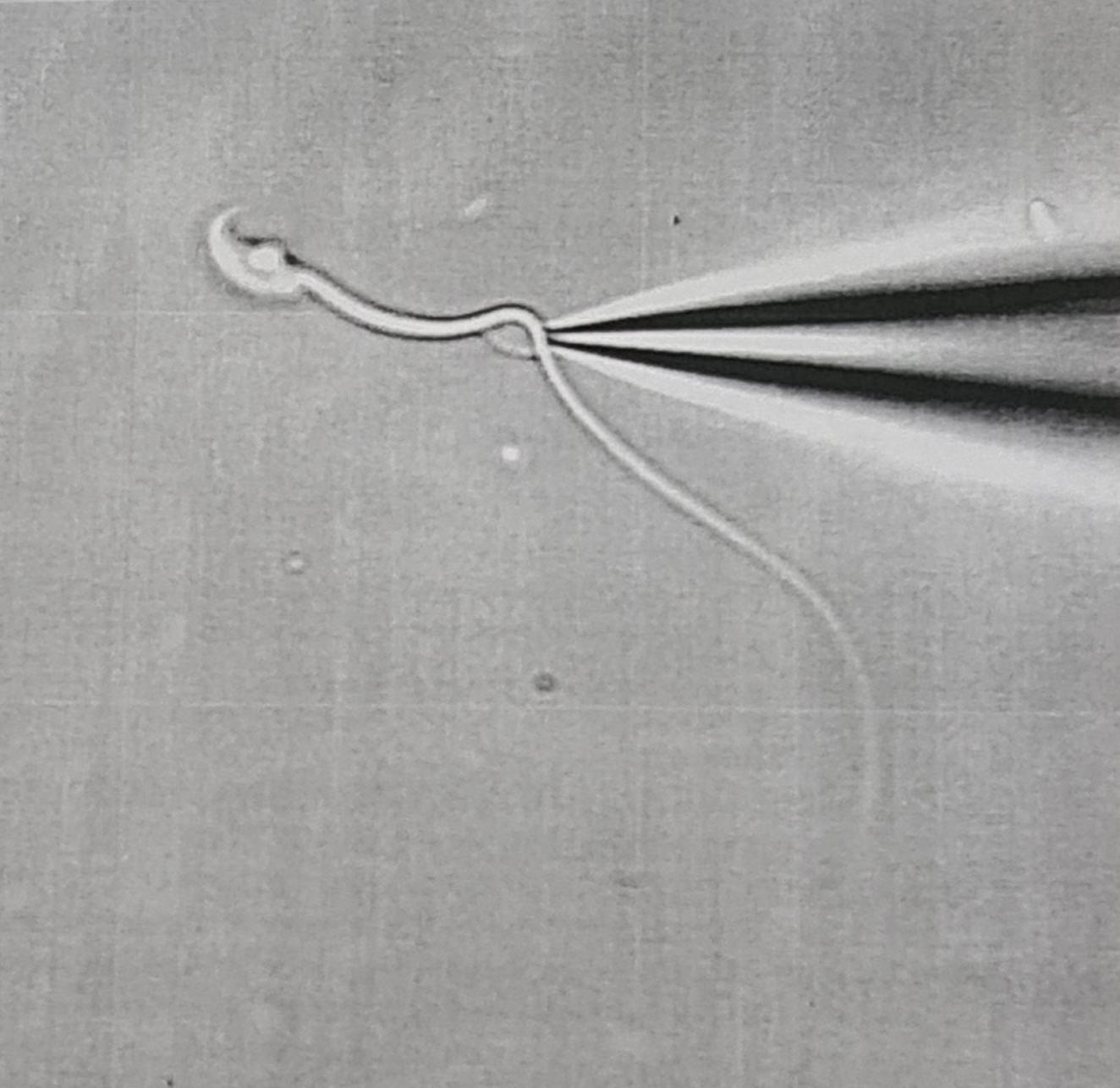
In the testis, spermatogenesis produces differentiated sperm cells yet unable to move and to interact with the oocytes. A maturation of the sperm cells is required in order to promote the fertilization process. Although this maturation process is initiated in the epididymis, in the female genital environment, sperm undergo a process essential for fertilization called capacitation, characterized by a change in the intracellular pH, membrane hyperpolarization and a rise in the intracellular calcium concentration. This process leads to the development of a hyperactivated motility and the ability for the spermatozoa to carry out the acrosome reaction. Both the hyperactivation and the acrosome reaction are physiological processes highly dependent on the sperm cell intracellular calcium concentration.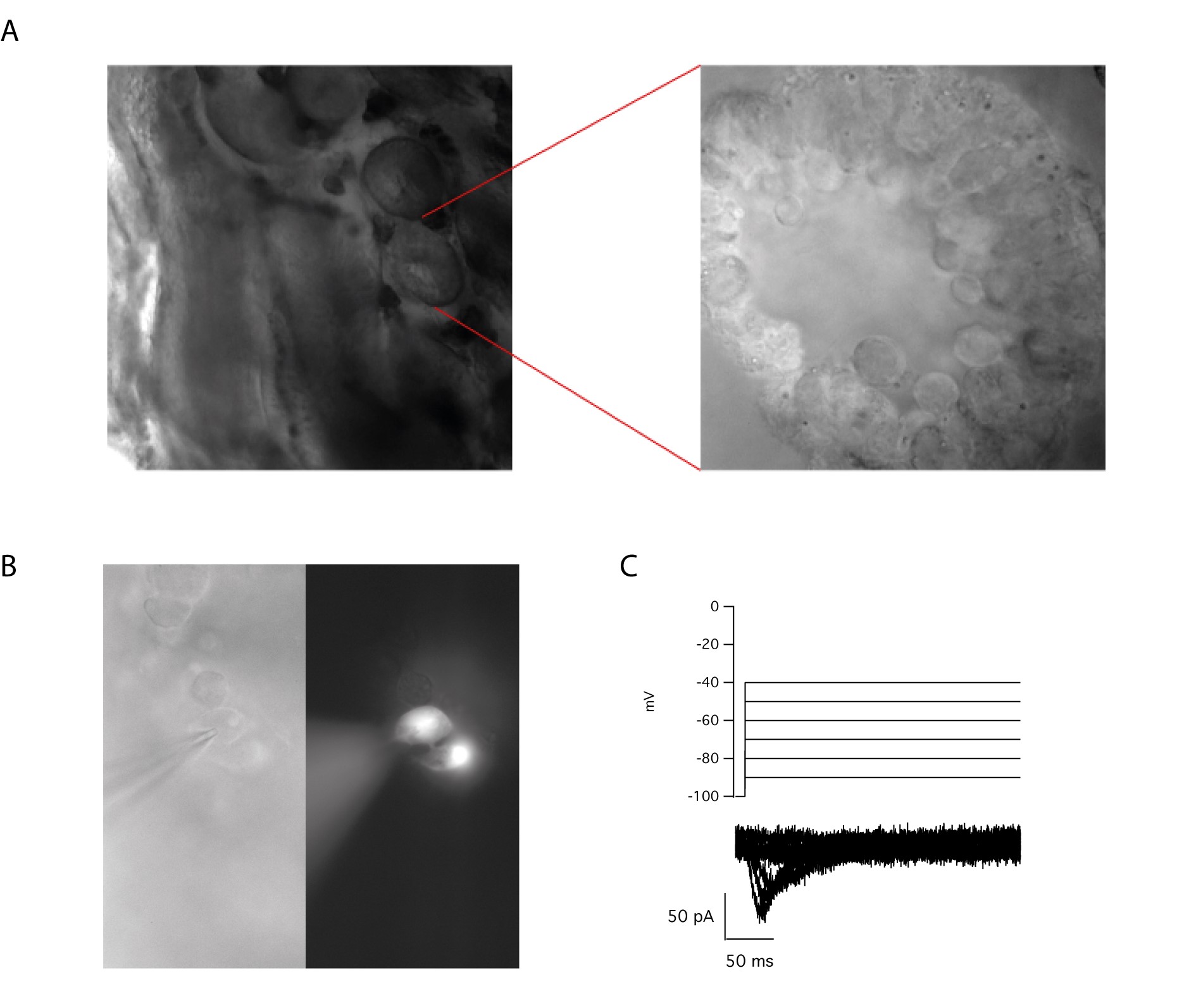
The spatio-temporal regulation of the calcium concentration in the spermatozoon is therefore of the utmost importance. The capacitation process has been extensively studied by Pascale Lybaert in collaboration with David Gall at ULB and during their research experiences in the USA. This research is in fact a continuation of P. Lybaert's thesis work and a project initiated during a 24-month scientific mission by Pascale Lybaert (2015-2017) in Celia Santi's laboratory at the Washington University. in St Louis paving the way for strong collaboration between our teams.We study the capacitation signaling cascade and the interactions of these 3 parameters (pH, membrane potential, [Ca2+] ) in the mice model and in human spermatozoa by measuring their variation using spectrofluorimetric techniques and by flow cytometry. The functional evaluation of down-stream physiological processes is performed by CASA sperm motility parameters measurements and acrosome reaction quantification.
We are also developing a mice model based on the Cre-Lox system to follow the variations of the intracellular calcium concentration using a sperm-expressed genetically-encoded calcium indicator.
and maturation in mammals are notoriously difficult to unravel, given the intricate anatomy and complex endo- and paracrinology of the testis. Ion channels and transporters are key elements in sperm–egg signaling, environmental sensing and are essential for fertilization, but little is known about their role in spermatogenesis. We have developed new procedure to obtain acute testis slices that will allow to study the evolution of the electrophysiological profile of germ cells during their maturation. Investigating these biological properties may provide critical insights for understanding stem cell biology and tissue development.
In the testis, spermatogenesis produces differentiated sperm cells yet unable to move and to interact with the oocytes. A maturation of the sperm cells is required in order to promote the fertilization process. Although this maturation process is initiated in the epididymis, in the female genital environment, sperm undergo a process essential for fertilization called capacitation, characterized by a change in the intracellular pH, membrane hyperpolarization and a rise in the intracellular calcium concentration. This process leads to the development of a hyperactivated motility and the ability for the spermatozoa to carry out the acrosome reaction. Both the hyperactivation and the acrosome reaction are physiological processes highly dependent on the sperm cell intracellular calcium concentration.

The spatio-temporal regulation of the calcium concentration in the spermatozoon is therefore of the utmost importance. The capacitation process has been extensively studied by Pascale Lybaert in collaboration with David Gall at ULB and during their research experiences in the USA. This research is in fact a continuation of P. Lybaert's thesis work and a project initiated during a 24-month scientific mission by Pascale Lybaert (2015-2017) in Celia Santi's laboratory at the Washington University. in St Louis paving the way for strong collaboration between our teams.We study the capacitation signaling cascade and the interactions of these 3 parameters (pH, membrane potential, [Ca2+] ) in the mice model and in human spermatozoa by measuring their variation using spectrofluorimetric techniques and by flow cytometry. The functional evaluation of down-stream physiological processes is performed by CASA sperm motility parameters measurements and acrosome reaction quantification.
We are also developing a mice model based on the Cre-Lox system to follow the variations of the intracellular calcium concentration using a sperm-expressed genetically-encoded calcium indicator.
(A) Cross-sections of seminiferous tubes are present on acute testis slices and allow to characterize different germ cell populations using the whole cell configuration of the patch clamp technique.(B) Injection of ALEXA FLUOR 488 through the recording pipette clearly demonstrates the existence of cell to cell communication as the fluorescent probe can diffuse from the recorded cell to its neighbour. (C) T-type calcium currents are present in immature sperm cells.
In the laboratory, we are also working on sperm channels and ion transporters. They are key components of sperm-egg signaling, environmental sensing, and are essential for fertilization, but little is known about their role in spermatogenesis.
The application of the patch-clamp technique to the mature sperm cell has opened new perspectives to the study of the different actors involved in the acquisition of fertility. We have successfully implemented a procedure that permits to obtain acute slices from mouse testis, which are suitable for electrophysiological recordings.
This innovative technique allows us to compare the ex vivo electrophysiological profile of spermatogenic cells at different stages of maturation to similar studies performed on isolated germ cells. The aim of this study is to determine which ion channels are present in immature sperm cells and could be important for their differentiation and when the ion channels that are essentials for capacitation (CatSper, SLO) are expressed and become functional during spermatogenesis.
The impact of endocrine disruptors on the expression of germ cells ion channels and their conductances will thereafter be evaluated to assess the potential detrimental effect of these agents on the male future fertility.
